Innovative gene therapy
Congenital retinal dystrophies are one of the most common causes of blindness in children and adolescents. There was not treatment until recently: the first gene therapy for hereditary retinal diseases, voretigene neparvovec (Luxturna), has been approved for use by the European Medicines Agency. Reimbursement in Belgium has now also been put in place. This new gene therapy is suitable for people with a hereditary retinal disease caused by mutations in the RPE65 gene.
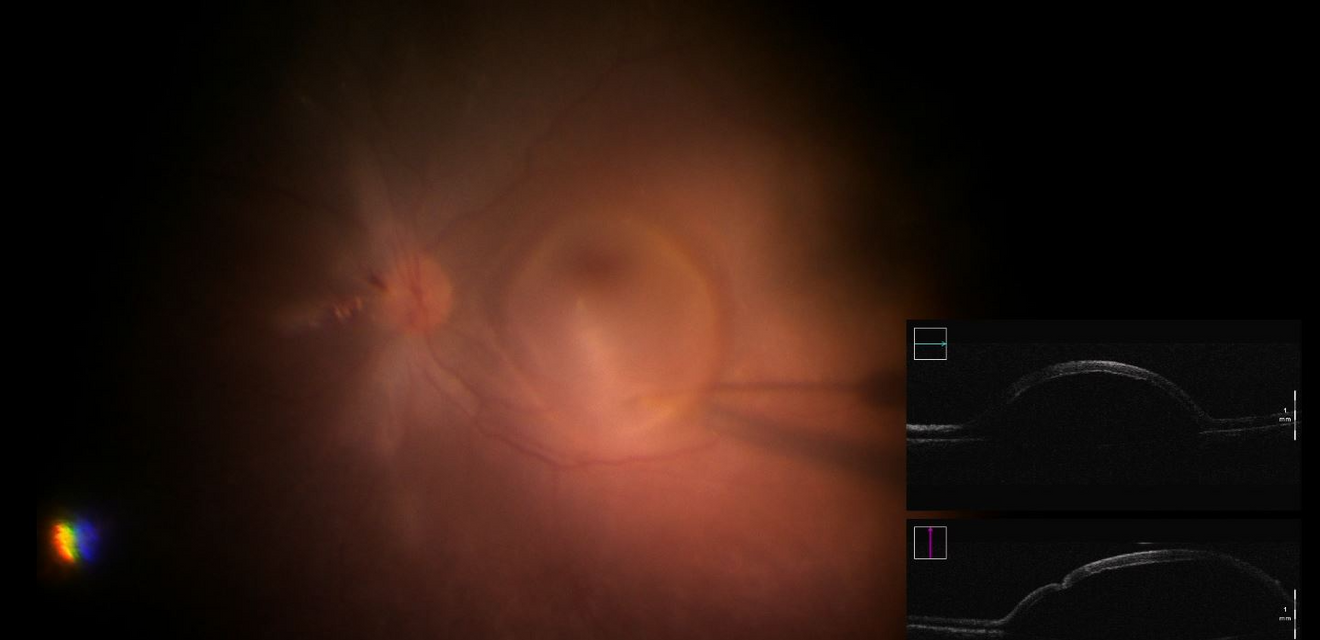
At UZ Leuven a first patient has been treated with the innovative gene therapy. Dr. Joachim Van Calster, vitreoretinal surgeon at UZ Leuven: “During this type of comples retinal surgery more than a billion viruses each with an intact RPE65 gene are injected under the retina. These genes are introduced into the body's own cells by means of a viral vector, where they replace the defective gene. This is done through a recent but complex technique, where the doctor works under the microscope and the vital parts of the eye are very close to, or even part of, the surgical procedure.”
If the disease is diagnosed in time, this new treatment can prevent further progression.Dr. Joachim Van Calster, vitreoretinal surgeon
Timely diagnosis
Given the progression of this disease, timely and correct diagnosis is of the utmost importance. The therapy is administered once and is only effective in case there are still enough living photoreceptors present. This new treatment can only prevent further progression if the disease is detected in time.